|
History -Chemical History of UCL1828 Edward Turner
Turner was only 29 when he was appointed. He thought that there was too much hypothesis and too little established fact in chemistry, and he set about establishing analytical methods by which atomic weights could be determined. The slide shows Turner's figures from 1833 and the IUPAC values which are presently accepted, and the agreement is remarkable. 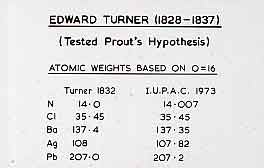 Atomic weights determined by Turner compared with modern values There was at the time a hypothesis (Prout's hypothesis) that all atomic weights were multiples of that of hydrogen, and if the atomic weight of hydrogen was one, then all the others should be integral numbers. Turner was sure of his figures for, for example, chlorine and he proclaimed that "Dr. Prout's hypothesis cannot be upheld". Turner wrote two books, "The Laws of Chemical Combinations", and, in 1827, the first organic text book, "Elements of Chemistry" and which went to 8 editions with Liebig as editor and gained a European reputation. The frontispiece of the 5th edition, published while he was Professor at UCL in 1834 is shown below (courtesy of Prof Peter Garratt). 
This page last modified 20 September, 2010 |

