An atomic H beam is easily produced by passing H 2 gas through a microwave discharge tube using a highly efficient microwave radiator oscillating at 2.45 GHz [1]. This radiator produces a 2H:H 2 dissociation yield as high as 90% at a flux of ~10 17 atoms/second, operating at pressures from ~0.1 - 2 torr [1]. The radiator is held between two water-cooled copper flanges to prevent overheating, and is mounted on a UHV flange to allow the atom beam to pass directly into the vacuum system.
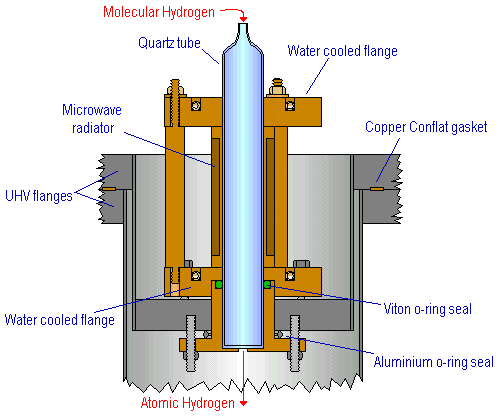
Cross-section through the Atomic Hydrogen Source
Once produced the H atoms are transported along a Teflon tube (~30 cm in length) at room temperature to a cooling block without significant recombination losses [2]. The cooling block, constructed from aluminium alloy, is held at ~100K by a commercial cryogenic cold-head. The atomic H beam is allowed to pass through this block, and is cooled via collisions with the aluminium oxide surfaces. Since the H+H recombination efficiency is low on aluminium oxide at ~100K [3], this reduces the translational kinetic energy of the beam without significant losses.
After leaving the cooling block, the atom beam is allowed to free-fly to the target chamber via the chopper chamber.
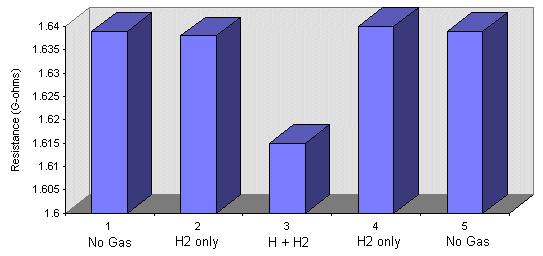
The H beam profile can be monitored using a semiconducting H atom detector. This is a small chip made from an amalgam of manganese and nickel oxides made at UCL. It has been shown that the resistance of this material changes when hydrogen atoms are chemisorbed on its surface [4]. Although it is sensitive to hydrogen atoms, it does not respond to molecular hydrogen. This detector provides a useful check that we are making hydrogen atoms in our hydrogen source, and also enable us to measure the beam profile and obtain an indication of its concentration. A typical response characteristic is demonstrated below: