
|
 |
Academic Staff - Dr Mark Green - Inorganic
Early Transition Metal Oxides
1. Electronic properties of early transition metal oxides.
There are many interesting ground states and phenomena unique to low dimensional materials, such as the magnetic spin-Peierls transition, as well as important theoretical predictions of high temperature superconductivity in doped spin ladder systems. In this research program, we are developing new low dimensional and/or conductive materials which constitutes a significant area of nanotechnology.
 |
Figure 1: The one-dimensional structure of dichlorobithiazole copper(II). |
One dimensional magnetic order has previously been largely confined to systems containing organic ligands, as their structures have a sufficiently large distance between chains, thereby creating true one dimensional character, such as dichlorobi(thiazole) copper (II) (Figure 1), where Cu is displayed in red. However, there are only a limited number of examples, sample synthesis can be difficult and substitutional chemistry to subtly alter properties is often not possible. There are a few examples of inorganic one dimensional magnets but these tend to have significant interchain interactions and do not show ideal behaviour. The research will focus on the synthesis of new metal oxides that contain a mixture of d 0 and d 1 ions. The geometry of the metal-oxygen co-ordination (mostly octahedral) of these two ions is normally extremely different; the d 0 ions adopting an irregular arrangement, such as in BaTiO 3 , with one long and one short bond, in contrast to regular arrangement seen in d 1 ions. The consequence of this is that in sufficiently complex structures, the d 0 and d 1 ions are situated on crystallographically distinct sites. This can have a profound effect on the magnetism, as one metal ions is magnetic (d 1 , s= 1/2) the second diamagnetic, and this can lead to novel magnetic ordering. This has been realised recently in our work on Nb 12 O 29 , which contains two unpaired electrons per (4x3) block of its crystallographic shear plane structure. It is found that one unpaired electron contributes to metallic conductivity, while one orders on a distinct site and creates one dimensional ordering, as shown in the figure 2.
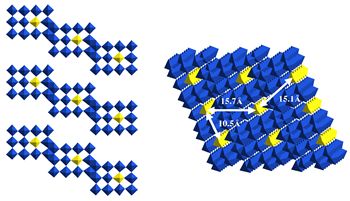 |
Figure 2 Structure of Nb 12 O 29 showing the (left) ordering of Nb 4+ (yellow) onto the corner-shared positions which shows (right) the formation of one dimensional chains which magnetically order at low temperature. |
Nb 12 O 29 is an important new example of an inorganic one dimensional antiferromagnet. The main difference between this and other examples is that the low dimensional character does not exclusively come from structural considerations, but it is through the nature of the charge ordering of the Nb 4+ ions. This allows a huge range of modifications to be performed in the alteration of number of localised electrons as well as the variation between structures. We propose to extend this work extensively into vanadium and titanium oxides, with emphasis on isolating new low dimensional magnets. Systems can also be envisaged which have metallic properties within the chains, separated by insulating blocks, forming nanowires, which could have important applications in the electronics industry. Synthesis will be performed using a number of methods, but makes specific use of flux growth and electrochemical crystallisation techniques.
This page last modified
9 August, 2010
|
 |




|
![]() +44 (0)20 7679 4650 - Copyright © 1999-2010 UCL
+44 (0)20 7679 4650 - Copyright © 1999-2010 UCL