
|
 |
Academic Staff - Professor Karl Hale - Synthetic Organic
 |
- Total Synthesis
- Pharmacologically-active natural products
- Retrosynthetic analysis
- New Synthetic methodology
- Amino Acid synthesis & Asymmetric synthesis
|
tel: +44 020 7679 4711 (office)
or 7679 4713 (lab)
fax: +44 020 7679 7463
internal phone: 24711 (office)
or 24713 (lab)
email: k.j.hale@ucl.ac.uk |
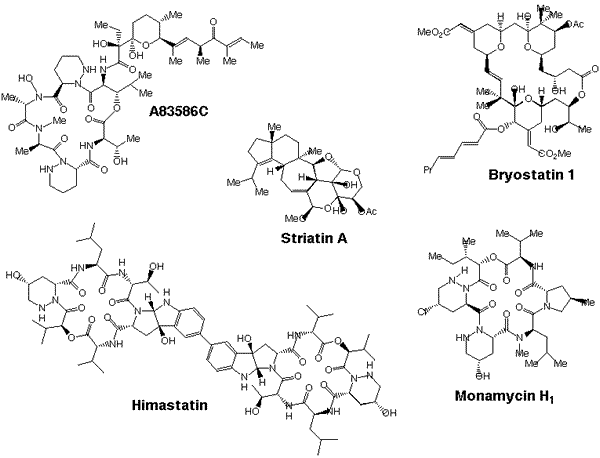
Research in the Hale group spans a number of areas, but is concerned primarily with the development of efficient, enantiospecific, synthetic strategies to complex natural products with important biological activity. Most of the target molecules selected for study are scarce in nature, and many operate by biological mechanisms that have yet to be fully defined. We develop total syntheses not only to improve the supply of these agents but also to gain access to novel analogues for biological evaluation. Synthetic analogues of important biomolecules often give valuable insights into how the parent compound functions as a therapeutically beneficial drug, and sometimes these analogues can themselves have enhanced pharmacological profiles. Current natural product targets of the Hale group include bryostatin 1 and himastatin, which are recently discovered anticancer drugs, and striatin A and monamycin H1 which are two antifungal antibiotics. Recent synthetic achievements include our first asymmetric total synthesis of the antitumour antibiotic A83586C.
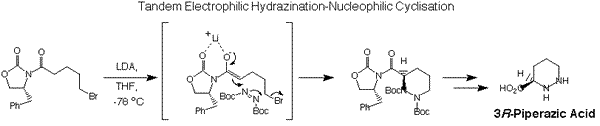
Quite frequently, our target molecule work necessitates us introducing valuable new synthetic methodology to attain the ultimate synthetic goals. One example of a novel reaction we pioneered for our A83586C project is the tandem electrophilic hydrazination-nucleophilic cyclisation shown below.
Selected Publications
- J. Hale and J. Cai. Asymmetric total synthesis of antitumour antibiotic A83586C. Chem. Commun., 1997 , 2319.
- K.J. Hale, J. Cai, and G. Williams. Total synthesis of 4-Epi-A83586C. Epimerisation in a macrolactamisation mediated by BOP and DMAP. Synlett, 1998 , 149.
- K.J. Hale, J.A. Lennon, S. Manaviazar, M.H. Javaid, and C. Hobbs. Asymmetric synthesis of the C(17)-C(27) segment of the antineoplastic macrolide bryostatin 1. Tetrahedron Lett., 1995 , 36, 1359.
- K.J. Hale, S. Manaviazar, and R. Tupprasoot. The synthesis and reactions of some novel 5,6-pyranoid glycals derived from D-fructose. Tetrahedron, 1997 , 53, 16365.
- Karl J. Hale, Vern M. Delisser, Li-Kuan Yeh, S. Andrew Peak, Soraya Manaviazar, and Gurpreet S. Bhatia, Synthetic Studies on the Azinothricin Family of Antibiotics. 3. Enantioselective Synthesis of a Hexapeptide Precursor for Antitumour Antibiotic A83586C. Tetrahedron Lett. , 1994 , 35 , 7685-7688.
- Karl J. Hale, J.A. Lennon, Soraya Manaviazar, and Muhammad H. Javaid, Asymmetric Synthesis of the C(17)-C(27) Segment of the Antineoplastic Macrolide Bryostatin 1. Tetrahedron Lett. , 1995 , 36 , 1359-1362.
- Karl J. Hale, Jiaqiang Cai, Soraya Manaviazar, and S. Andrew Peak, Synthetic Studies on the Azinothricin Family of Antibiotics. 4. Enantioselective Synthesis of the Northern Half of Antitumour Antibiotics A83586C and Citropeptin. Tetrahedron Lett. , 1995 , 36 , 6965-6968.
- Karl J. Hale, Jiaqiang Cai, Vern Delisser, Soraya Manaviazar, S. Andrew Peak, Gurpreet S.Bhatia, Timothy C. Collins, and (in part) Neha Jogiya, Enantioselective Synthesis of (3R)- and (3S)-Piperazic Acids. The Comparative Unimportance of DMPU Mediated Retro-Hydrazination. Tetrahedron , 1996 , 52 , 1047-1068.
This page last modified
9 August, 2010
|
 |




|



