ABSORPTION SPECTROSCOPY
|
ABSORPTION SPECTROSCOPY
|
We are currently undertaking a programme to record absolute VUV photoabsorption cross-sections for molecules in the gas phase. In particular, we plan to record such spectra of the PAHs and related molecules. Some preliminary measurements have already been performed, indicating that (i) the spectra contain bands with large cross-sections, and (ii) simple substituents such as the methyl group can lead to significant spectroscopic shifts, e.g. ~100 meV in the case of 2-methyl naphthalene. These preliminary data are presented here.
Briefly, polycyclic aromatic hydrocarbons are molecules built up of benzene rings which resemble fragments of single layers of graphite. They have planar structures and come in a wide variety of shapes and sizes.
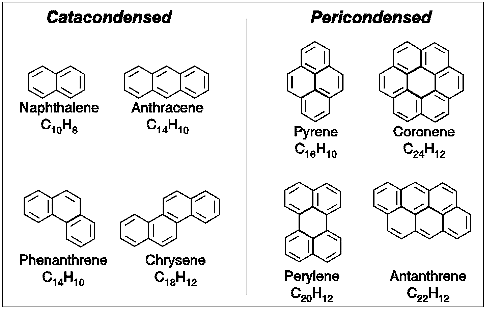 Some typical PAH structures.
Some typical PAH structures.
On Earth these compounds are quite common, being formed during incomplete
combustion of almost any kind of organic material hence they are continually
being released into the environment [1]. They are
present in coal extracts, internal combustion engine exhaust fumes, cigarette
smoke, soil, marine sediments, soot, smoke from wood burning, and even fried
or grilled food! Since some of the PAHs are known carcinogens [2]
their presence in the environment (particularly in food) is a cause of concern
to health authorities.
Astronomical observations of the interstellar medium (ISM) have revealed three main sources of mystery [3]:
Recently, the PAHs have been shown to be good candidates for each of these
observations. Therefore, it has been proposed that PAHs are present in the
interstellar medium (ISM) in relatively high abundances (see, for example
[4, 5]). However, although the PAHs seem to be good candidates
to explain some of these phenomena, not one single PAH molecule has yet been
identified in the ISM. Current thought is that if PAHs are present,
they may be in modified form, e.g. ionised or protonated [6].
Two main mechanisms have been proposed to account for the possible formation
of PAHs in the ISM. First, collisions between dust grains (thought to
consist of graphite and/or silicates) could fracture the graphite planes thus
releasing free PAHs [7]. Secondly, PAHs could Îgrowâ
from reactions between smaller unsaturated hydrocarbon molecules and radicals
in the remnants of carbon rich stars [8]. Once formed
the PAHs would be remarkably stable, and would resist dissociation from UV absorption
(unlike most other polyatomic molecules in the ISM) since they are extremely
efficient at rapidly re-emitting the absorbed energy at infra-red wavelengths
[7].
The UV photoabsorption spectroscopy of the PAHs is also known to be related
to that of benzene since the delocalised pi-orbitals are dominant, hence they
have very strong absorption bands between 4-7 eV. These bands occur in
the region of the astronomically observed UV extinction bump (~220 nm »
5.6 eV), hence it is possible that PAHs may be the cause of this mysterious
astronomical feature [9]. Therefore, the accurate
measurement of photoabsorption spectra of the PAHs has become an experimental
goal in recent years. However, reliable measurements of absolute cross-sections
remain sparse due to the difficulties involved in handling PAH samples.
For this reason, members of the Centre for Cosmic Chemistry
and Physics have attempted to measure accurate cross-sections of some simple
PAHs in conjunction with the Molecular
Physics Laboratory at UCL.
VUV photoabsorption spectra of some small PAHs were recorded , using a photoabsorption
apparatus attached to the synchrotron light source at the UK Daresbury
Laboratory. Details of this instrument may be found in [10].
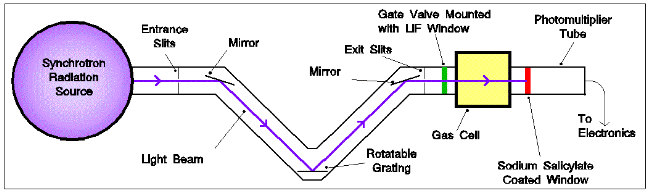
Experimental apparatus used to record photoabsorption cross-sections of
PAHs
Briefly, the synchrotron radiation enters and leaves an absorption cell through LiF windows. Transmitted radiation passes into a photon-monitoring section for detection with a photomultiplier tube. The absorption path length of the cell is 16 cm, and the spectral range available for this experiment extended from about 5 eV (250 nm) to the LiF cut-off at 11.2 eV (110 nm). This was covered in sections of about 20 nm, each scanned in steps of 0.05 nm.
At each wavelength in the limited (20 nm) spectral range, the radiation transmitted
through the sample, the sample pressure, and the synchrotron beam ring current
were recorded. The sample cell was then evacuated, and the radiation transmitted
through the empty cell was measured along with the ring current across the
same spectral range. The transmitted radiation was normalised to a constant
ring current before analysis using the Beer-Lambert law.
Several different PAHs were investigated using the spectrometer with a variable
degree of success. The main difficulty experienced was that the PAHs,
being solids at room temperature, had very low vapour pressures hence it was
difficult to obtain sufficient sample pressures within the gas cell. When
the samples were heated to increase the vapour pressure, the hot vapours evolved
from the crystals were found to condense within the cell and subsequently resisted
attempts to remove them. However, despite these problems reliable data
were obtained for some of the smaller compounds.
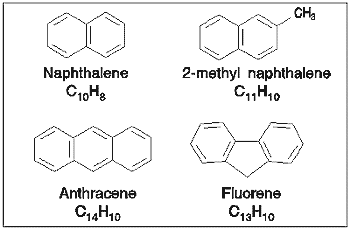
The spectra obtained for these compounds are shown below; absolute cross-sections
were obtained for all of the compounds except anthracene, for which relative
cross-sections were obtained. Due to difficulties in measuring low pressures
accurately within the gas cell, the estimated error in the cross-sections
recorded for these molecules was ±30%. Some of the data shown below
have been published [11].
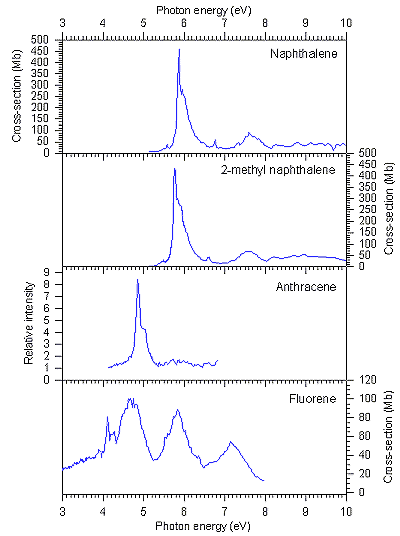
The shapes of the observed spectra are in agreement with those of previous
measurements (where available: naphthalene [12]; anthracene
[13]). The spectra of naphthalene, 2-methyl naphthalene
and anthracene were all observed to have strong absorption bands around 5-6
eV; this was in the energy region of the astronomically observed UV extinction
bump, hence these molecules may be contributors to that feature. The strong
bands represented transitions between the p-orbitals of the molecules, and were
reminiscent of the benzenoid 1E1u transition. However,
the spectrum of fluorene was observed to be different to the others, showing
that absorption spectra of the PAHs are sensitive to the presence of non-aromatic
groups. Nevertheless, although the overall shape of the fluorene spectrum was
different, it still showed strong absorptions in the region of the UV extinction
bump hence it may be present in the interstellar medium. Fluorene is particularly
interesting, since its structure can be thought of as a fragment of a fullerene
molecule, C60, popularly known as a "buckyball".
The data recorded for naphthalene in this work are in good agreement with previous
measurements, giving confidence in the data recorded for the other PAHs studied.
This suggests that reliable photoabsorption spectra could be obtained for other
small PAHs using this apparatus in the future. However, to obtain data
for the larger species would require modifications to the gas cell to allow
the entire system to be heated to increase the vapour pressures of the samples
without their condensation inside the spectrometer. Designs for such a
device are currently being contrived so that further investigation into the
spectroscopy of the PAHs can be performed to provide more data of astrophysical
interest.
[1] W. Schmidt, in Polycyclic Aromatic Hydrocarbons
and Astrophysics, eds. A. Leger et al (D. Reidel Publishing
Co., Dortrecht, 1987) pp. 149-164.
[2] H. Busch (ed.), The Molecular Biology of Cancer
(Academic, New York, 1974).
[3] D.A. Williams and S.D. Turner, Q. J. Roy. Astron.
Soc. 37 (1996) 565.
[4] L.J. Allamandola, A.G.G.M. Tielens and J.R. Barker,
Astrophys. J. 290 (1985) L25.
[5] D.J. Cook et al, Nature 380
#21 (1996) 227.
[6] D.M. Hudgins, S.A. Sandford and L.J. Allamandola, J.
Phys. Chem. 98 (1994) 4243.
[7] A. Leger and L. D'Hendecourt, in Polycyclic Aromatic
Hydrocarbons and Astrophysics, eds. A. Leger et al (D. Reidel
Publishing Co., Dortrecht, 1987) pp. 223-254.
[8] D.A. Howe, J.M.C. Rawlings and D.A. Williams, Adv.
At. Mol. Opt. Phys. 32 (1994) 187.
[9] C. Joblin, A. Leger and P. Martin, Astrophys. J.
393 (1992) L29.
[10] N.J. Mason, J.M. Gingell, J.A. Davies, H. Zhao, I.C. Walker and
M.R.F. Siggel, J. Phys. B 29 (1996) 3075.
[11] J.M. Gingell, Faraday Discussion 109 (1998).
[12] F. Salama and L.J. Allamandola, Astrophys. J. 395
(1992) 301.
[13] T. Kitagawa, J. Mol. Spectrosc. 26 (1968)
1.
 Comments/enquiries:
a.wolff@ucl.ac.uk
Comments/enquiries:
a.wolff@ucl.ac.uk