|
History -Chemical History of UCLThe Discovery of Argon
On April 19 th 1894 Ramsay heard Lord Rayleigh lecture to the Royal Society, when he pointed out that nitrogen isolated from the air had a density slightly higher than that of nitrogen prepared from chemical sources. A litre of pure nitrogen gas generated from a chemical reaction weighed 1.2505 g. On the other hand, a litre of nitrogen gas generated from air by removing oxygen, carbon dioxide, and water vapour weighed 1.2572 g (at the same temperature and pressure). Rayleigh thought that this might be due to the presence of a light impurity in the former ( Rayleigh's paper is available online ). But Ramsay thought that it might be due to the presence of a heavy impurity in the 'atmospheric' nitrogen. He was an enthusiast for the Newland/Mendeleev Periodic Table and we have Mendeleev's signature in one of our books of visitors. He thought that there might be an unrecognised new element hiding in the air, and that there might be room for this in a new Group at the end of the Periodic Table.
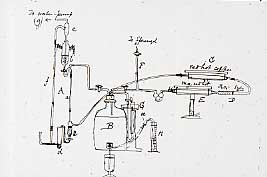
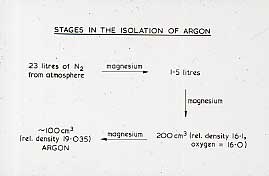
When examinations had finished, Ramsay set about attempting to isolate this 'impurity'. Ramsay's Apparatus for Isolating ArgonHe repeatedly passed nitrogen from the air over red hot magnesium, which reacted to form magnesium nitride, and as the volume decreased, the density rose. 22 Litres of the gas with a density of 14 were reduced to 1.5 l with a density of 16.1, and then finally to a residual 290 cm 3 with a density of 16.1 and then to 290 cm 3 with a density of 19.95, and which would no longer react with magnesium. Measurement of the specific heat showed it to be monatomic, and therefore the atomic weight was 39.9, and it fitted into the Periodic Table between chlorine and potassium as the first member of a new Group. With the advice of a colleague from the Classics Department, he called this gas argon, after the name argon given in the Greek Old Testament to describe 'the workers who stand idle in the market place'.
These experiments were later described by Lord Rayleigh in an informal but fascinating evening lecture . Ramsay's paper on the discovery is also available online . (We are grateful to Prof Carmen Giunta for putting the above papers online).
This page last modified 20 September, 2010 |
||||||||||||||||||