|
History -Chemical History of UCLThe Discovery of Neon and Other Gases
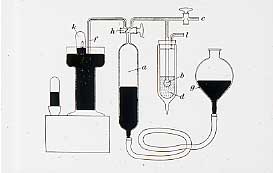
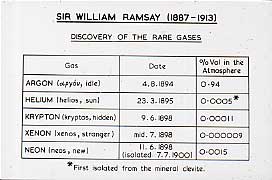
In 1895, Henry Meirs, at the British Museum, told Ramsay that, on heating, a mineral cléveite gave off a gas that Meirs though might be nitrogen. Ramsay thought that it might be a compound of argon. He sent out his technician to a minerals dealer for a specimen of cléveite and, in two days, he showed that it was a new inert gas, helium, the spectrum of which Sir William Crookes had observed from the light of the sun in 1868. With an atomic weight of 4, it fits between hydrogen and lithium, in the same group as argon. They were now faced with an almost insuperable problem: they have found the first and the third member of the Group, and now need to find the intermediate member, and Ramsay says: "Here is a supposed gas, endowed no doubt with inert properties, and the whole world to find it in". After 2 years, he decided that it might be hiding in the atmosphere. At the Royal Institution in Piccadilly, Dewar had liquefied air in 1872. Ramsay and his student, Maurice Travers , cooled bulbs of argon in liquid air, and separated off the uncondensed portion. Under an electrical discharge it gave blaze of crimson light, and they called it Neon, the newcomer.
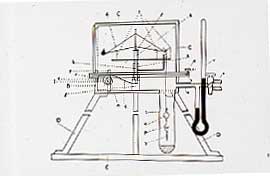
In 1898, they cooled the crude neon with liquid hydrogen and isolated the forth and fifth members of the series, Krypton (the hidden one), and Xenon (the stranger) which was present in air to the extent of 1 in 10 8 . We have many of Ramsay's original gas tubes and a direct vision spectrometer which he used to observe the discharge which they gave, and these tubes will still show their discharge with a Tesla coil. We hope to include photos of these at some later stage. Rutherford had shown that thorium gave what he called an "emanation". In 1900, Ramsay and Soddy showed that this was the sixth rare gas, Radon. Whytlaw Gray determined the density on a volume of gas of 3.2 x10 -8 cm 3 !! We still have fragments of the silica frame of the balance.
This page last modified 20 September, 2010 |